Transition metals – the most popular catalysts
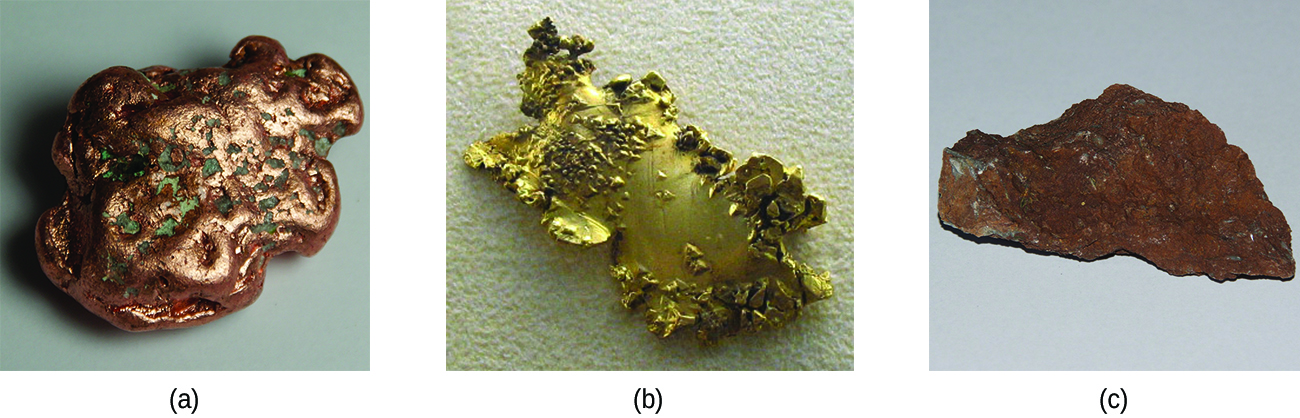
Transition metals include nickel, iron, and chromium that contain valence electrons in two shells instead of one. Valence electrons are electrons responsible for the chemical components of an atom. These metals are excellent metal catalysts because they take and lend electrons from different molecules. Catalyst handling companies use transition metals to speed up chemical reactions.
Effect of Catalysts
Catalysts work using catalytic pathways involved in a chemical reaction. They enhance the rate of collisions that occur between reactants without changing their chemical or physical components. They provide an alternative, low energy pathway for the reaction to happen. Catalysts influence the conversion rate of reactants by providing chemical reactants with a low energy transition path.
Transition Metals
Many people confuse transition metals with d-block elements. Although transition metals are categorized under d-block metals in the periodic table, not all d-block metals are identified as transition metals. Zinc and scandium are not transition metals despite being d-block elements. A d-block element must contain a moderately filled d-orbital for it to be identified as a transition metal.
Why Transition Metals Are Good Catalysts
The main reason why transition metals make good catalysts is because they can withdraw or lend electrons from the reagent. However, this is mainly determined by the nature of the reaction. Transition metals make good catalysts because of their abilities to exist in various oxidation states, form complexes with reagents, and be a good source for electrons.
Transition Metals as Electron Accepter and Donor
Scandium ion Sc3 is not a transition metal and does not have d-electrons. Zinc ion Zn2+ has full d-orbital and is not considered a transition metal. Transition metals have flexible and interchangeable oxidation states. They must also have d-electrons to spare. Copper has variable oxidation states including Cu2+ and Cu3+. These qualities makes it a perfect example of a transition metal. The metal is able to facilitate the exchange of electrons because of the incomplete d-orbital. Transition metals make the best catalysts because they can effortlessly give and take in electrons. The ability of the metal to create chemical bonds is what is referred to as the oxidation state.
Transition metals work by creating complexes with the reagent. These metals can take up excess electrons density and speed up chemical reactions. Catalysts play important roles in various industrial processes because they speed up chemical reactions. Transition metals are some of the most popular catalysts used in chemical processes.